 As cells age, they accumulate ‘trash’: misfolded and aggregated proteins, oxidized membranes, and even dysfunctional organelles. Lest the cell drown in its own waste, some kind of garbage disposal is necessary. Fortunately, eukaryotic cells have evolved a set of pathways, collectively termed “autophagy” (derived from the Greek words for “eating oneself”), for recycling unwanted components.
As cells age, they accumulate ‘trash’: misfolded and aggregated proteins, oxidized membranes, and even dysfunctional organelles. Lest the cell drown in its own waste, some kind of garbage disposal is necessary. Fortunately, eukaryotic cells have evolved a set of pathways, collectively termed “autophagy” (derived from the Greek words for “eating oneself”), for recycling unwanted components.
Autophagy is particularly important in non-dividing cells such as neurons, which can’t simply dilute out cellular detritus by growing faster than the trash builds up. Accordingly, defects in autophagy are linked to age-related neurodegenerative diseases such as Alzheimer’s and ALS. Conversely, we are increasingly finding that enhancing autophagy can help to prevent such diseases, as well as mitigating age-related deterioration more generally.
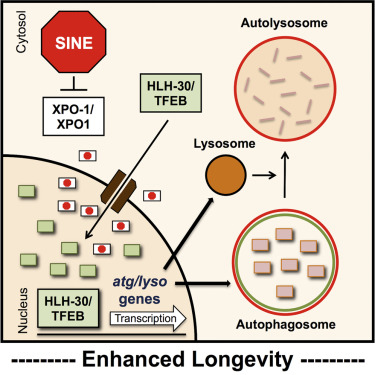
Consistent with this paradigm, new work from Louis Lapierre‘s lab at Brown University shows that stimulating autophagy can increase lifespan in model organisms and even protect against neurodegeneration in a fly model. While this is not the first indication that boosting autophagy slows aging, the authors of this paper use a clever new way to achieve this end: they increased the nuclear accumulation of TFEB, a transcription factor responsible for expression of autophagic factors, by blocking its export from the nucleus.
In initial experiments, the authors interfered with export function through a genetic manipulation, but they subsequently showed that pharmacological inhibition of export using compounds known as SINEs (selective inhibitors of nuclear export) had the same effect: reduced nuclear export led to higher levels of TFEB in the nucleus, which in turn increased transcription of autophagy proteins and boosted the activity of the pathway.
They went on to show that SINEs could boost autophagic activity in a fly model of ALS, as well as in cultured human cells. The fact that the drug works in multiple, evolutionary distant organisms indicates that the targeted mechanism is conserved (i.e., rather than an idiosyncratic feature of a single species). Furthermore, in the ALS flies, the intervention significantly ameliorated neurodegeneration, implying that this approach could in principle be applied to treating some of the most devastating diseases associated with aging.
Also important from the standpoint of translational medicine is the identification of what the senior author called “a new and conserved entry point” into an important pro-longevity pathway. Autophagy is under the control of the TOR pathway, and inhibition of TOR both activates autophagy (in part by promoting nuclear localization of TFEB) and extends lifespan. However, as we discussed here recently, chronic inhibition of TOR runs the risk of deleterious side effects, and it would be ideal to target the relevant downstream effectors of TOR inhibition (i.e., the factors that are directly responsible for extension of longevity) without knocking down the entire pathway for an appreciable fraction of the adult lifespan.
SINEs fit the bill: they activate autophagy and extend lifespan, but (as the authors show) they leave the activity of the TOR pathway untouched. Notably in this regard, SINEs are already being tested as anti-cancer drugs, and previous work has demonstrated their preclinical efficacy as well as their safety and tolerability in human subjects. Assuming that these early results hold, SINEs represent promising candidates for treating or even preventing neurodegeneration and other age-related diseases by activating autophagy.
Silvestrini et al. “Nuclear Export Inhibition Enhances HLH-30/TFEB Activity, Autophagy, and Lifespan.” Cell Reports, Volume 23 , Issue 7, pp. 1915 – 1921 (2018). DOI: 10.1016/j.celrep.2018.04.063
